Tonix is identifying, researching and developing promising therapies which have the potential to address unmet needs of patients with rare diseases and which may qualify for Orphan Drug Designation (ODD) by the FDA.
Prader-Willi Syndrome
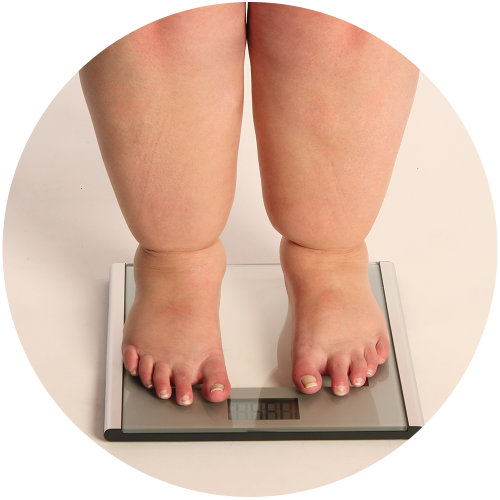
Prader-Willi syndrome is recognized as the most common genetic cause of life-threatening childhood obesity1 and affects males and females with equal frequency and all races and ethnicities. The hallmarks of Prader-Willi syndrome are lack of suckling in infants and, in children and adults, severe hyperphagia, an overriding physiological drive to eat, leading to severe obesity and other complications associated with significant mortality. There is currently no approved treatment for either the suckling deficit in babies or the obesity and hyperphagia in older children associated with Prader-Willi syndrome.
1 Foundation for Prader-Willi Research (fpwr.org)