About Us
Tonix is a biotechnology company where scientific breakthroughs begin with respecting the patient’s lived experience.
Our pipeline responds to the call for solutions to complex, chronic and sometimes invisible conditions with scientific rigor, integrity, and purpose. We are dedicated to developing therapies for conditions that defy easy answers, including nociplastic pain, immunology / immuno-oncology, and infectious diseases. We aim to develop impactful treatments that make patients feel seen and heard, reducing the limitations these conditions impose, enabling people to expect more, and be energized by possibility. At Tonix, we listen, we develop, we deliver – so that patients feel seen, heard, and empowered to live fully again.

Central Nervous System
Tonix’s CNS pipeline includes TNX-102 SL (cyclobenzaprine HCl sublingual tablets), in development for the treatment of acute stress disorder (ASD). Tonix has active IND’s (Investigational New Drug) applications for: multi-site pain associated with Long COVID (formally known as post-acute sequelae of COVID-19 [PASC]), posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), and agitation in Alzheimer’s disease (AAD).
Tonix’s CNS pipeline also includes active IND’s (Investigational New Drug) applications for: multi-site pain associated with Long COVID (formally known as post-acute sequelae of COVID-19 [PASC]), posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), and agitation in Alzheimer’s disease (AAD).
Infectious Disease
Tonix’s lead infectious disease product candidate, TNX-8012, is a live horsepox virus vaccine for percutaneous administration in development to protect against smallpox and mpox. Tonix will continue work on this live virus vector technology as a platform for rapid response to new pathogens.
Rare Diseases
TNX-2900, Tonix’s proprietary potentiated intranasal oxytocin, is in development for the treatment of Prader-Willi syndrome (PWS), the most common genetic cause of life-threatening childhood obesity. TNX-2900 has been granted Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation in the US by the FDA for the treatment of PWS. The FDA has cleared the Investigational New Drug (IND) application for TNX-2900 to progress into Phase 2 development.
Our Mission, Vision, and Core Values
Our Mission: Committed to improving health by inventing, developing and delivering impactful solutions, through robust in-house capabilities and creative collaborations, to address important unmet needs
Our Vision: We envision a world where people living with chronic and complex conditions are seen, heard and empowered to thrive
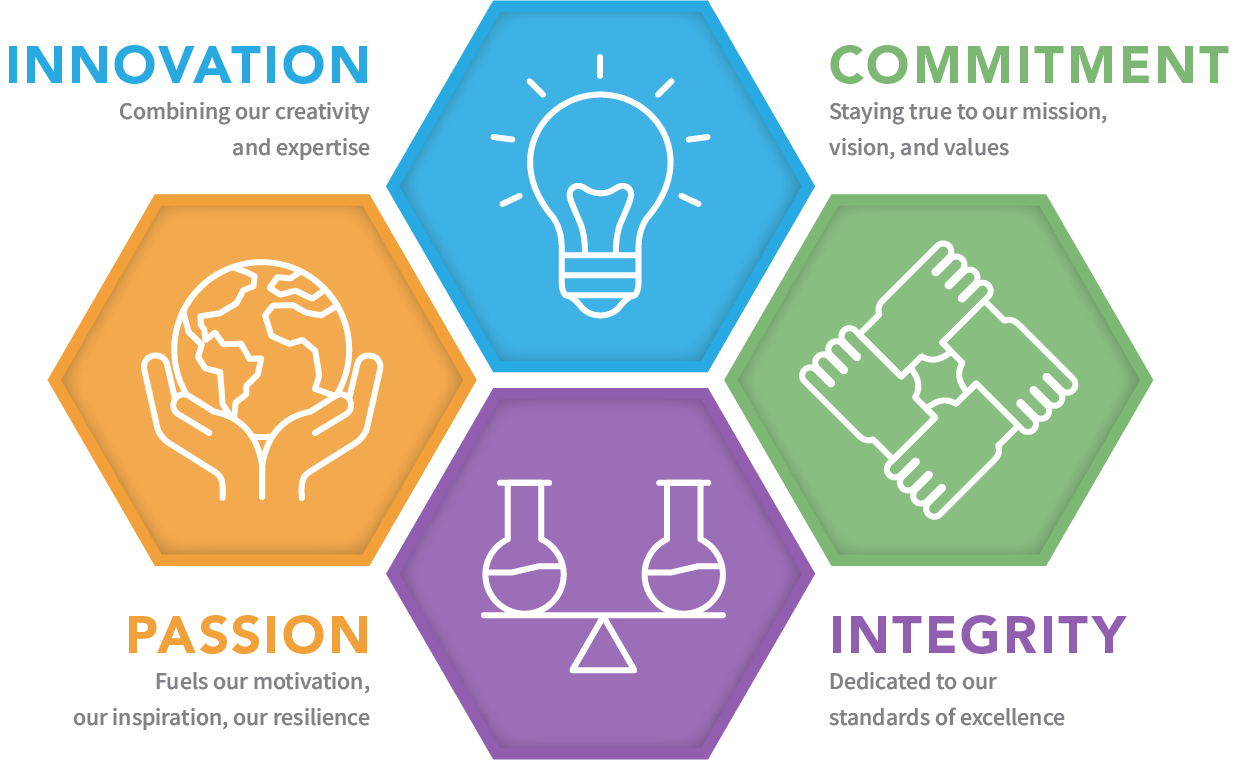
Our Core Values: Our values reflect our principles and define our culture as we strive to succeed in our mission and vision
Our Mission, Vision, and Core Values
Our Mission: Committed to improving health by inventing, developing and delivering impactful solutions, through robust in-house capabilities and creative collaborations, to address important unmet needs
Our Vision: We envision a world where people living with chronic and complex conditions are seen, heard and empowered to thrive
Our Core Values: Our values reflect our principles and define our culture as we strive to succeed in our mission and vision

Tonix Pharmaceuticals is a publicly traded company and is listed on the NASDAQ exchange under the ticker TNXP.
