About Us
Tonix is a biopharmaceutical company focused on developing novel therapeutics to prevent and treat central nervous system disorders, immunology conditions, infectious diseases, and rare diseases.
Tonix’s development portfolio is focused on central nervous system disorders. Tonix’s priority is to submit a New Drug Application (NDA) to the FDA for TNX-102 SL (cyclobenzaprine HCl sublingual tablet), which has completed two statistically significant Phase 3 studies for the management of fibromyalgia. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition, and acute stress disorder (ASD). Our pipeline is also comprised of immunology, rare disease, and infectious disease product candidates. Tonix’s immunology portfolio includes biologics to address organ transplant rejection, autoimmunity, and cancer. Our rare disease portfolio includes therapeutics to treat rare genetic conditions. Tonix’s infectious disease portfolio includes a live virus vaccine platform.
Central Nervous System
Tonix’s lead CNS candidate, TNX-102 SL1 (sublingual formulation of cyclobenzaprine) has completed two positive Phase 3 studies for the management of fibromyalgia, a disorder characterized by chronic musculoskeletal pain, sleep deprivation, fatigue and mood changes. TNX-102 SL is also being developed for Long COVID and acute stress disorder (ASD)
Infectious Disease
Tonix’s lead infectious disease product candidate, TNX-8012, is a live horsepox virus vaccine for percutaneous administration in development to protect against smallpox and monkeypox. Tonix will continue work on this live virus vector technology as a platform for rapid response to new pathogens.

Rare Disease
TNX-2900, Tonix’s proprietary potentiated intranasal oxytocin, is in development for the treatment of Prader-Willi syndrome (PWS), the most common genetic cause of life-threatening childhood obesity. TNX-2900 has been granted Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation in the US by the FDA for the treatment of PWS.
Our Mission, Vision, and Core Values
Our Mission is to improve population health by inventing and developing innovative therapies and vaccines, through broad in-house capabilities and creative collaborations, to help address important unmet needs. We strive to do this by using our integrated development engine to advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential.
Our Vision is to be a leader in providing novel drug therapies and vaccines to improve population health around the world.
Our Core Values reflect our principles and define our culture as we strive to succeed in our mission and vision.
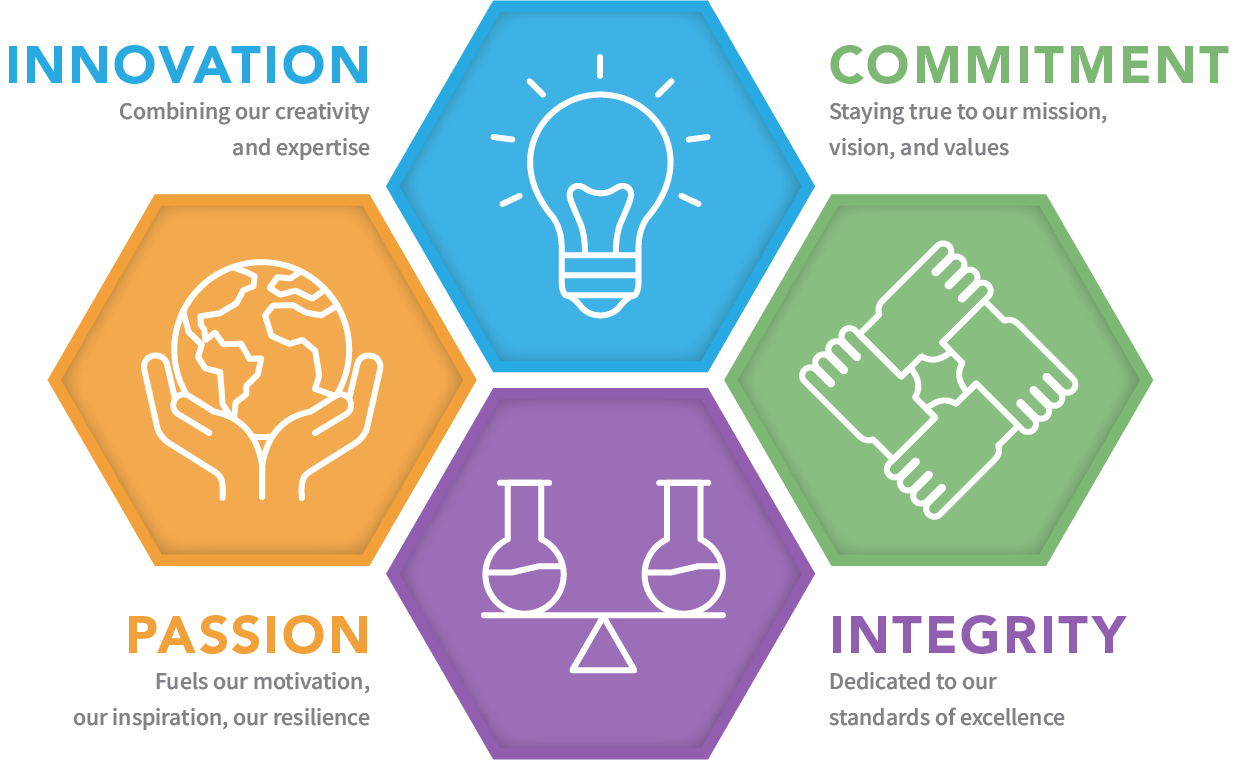
Our Mission, Vision, and Core Values
Our Mission is to improve population health by inventing and developing innovative therapies and vaccines, through broad in-house capabilities and creative collaborations, to help address important unmet needs. We strive to do this by using our integrated development engine to advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential.
Our Vision is to be a leader in providing novel drug therapies and vaccines to improve population health around the world.
Our Core Values reflect our principles and define our culture as we strive to succeed in our mission and vision.

Tonix Pharmaceuticals is a publicly traded company and is listed on the NASDAQ exchange under the ticker TNXP.
